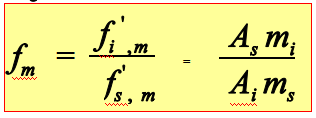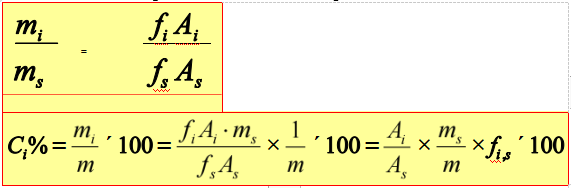Experiment Objectives
1. Learn Gas Chromatograph basic structure and basic operation.
2. Learn the separation principle of column, and affection factors of resolution
3. Learn how to calculate resolution.
4. Learn the qualitative analysis based on retention time and internal standard quantitative analysis method.
Gas Chromatograph
Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance or separating the different components of a mixture.
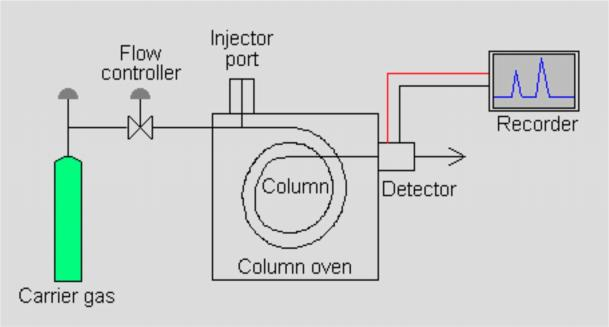
Fig. 1 Gas Chromatograph Structure Diagram
Instrument Structure
Carrier gas
Carry sample to separate through the column, arrive in detector.
Carrier gas used for GC must be chemical inert.
High purity, need remove moisture and impurities if necessary. Commonly type: Nitrogen gas, helium, argon, hydrogen, carbon dioxide The carrier gas selection mainly considers to match the type of detector.
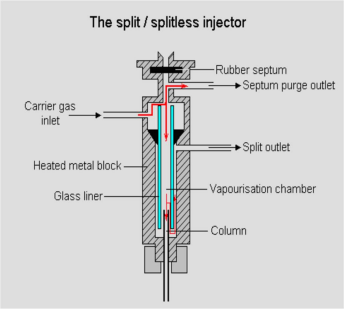
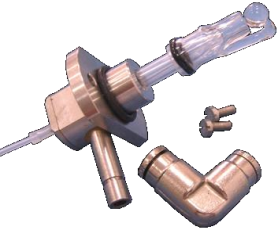
Inlet
Sampling and vaporize sample to gas before come to column.
Fig.2 Inlet diagram
Temp. Setting: 50 ℃ more than highest boil point compound.
Packed inlet sampling volume: 1mL
Capillary inlet sampling volume: 1ul
Split ratio: Depend on the density of sample and column capacity
Column
Packed Column:
Chemical inert and uniform filler
The filler surface of column is coated with a liquid stationary phase
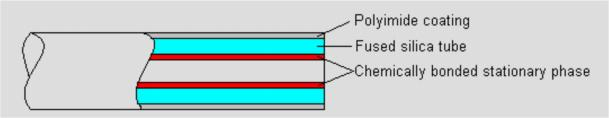
Most packed columns are 1.5 to 10m in length and 2 to 4 mm in inner diameter Capillary column
Fused silica capillary column
Inner diameter less than 1 mm, length from a few meters to one hundred meters
Fig.3 Capillary column structure
Column oven temperature
Temperature control error within 0. 1°C.
The appropriate column temperature is slightly higher than the average boiling point of the sample.
Reducing the column temperature can increase the resolution and extend the analysis time.
Improve the column temperature, speed up the peak out, and reduce the degree of separation.
If the boiling points of the components in the sample are very different, a programmed temperature increase method should be adopted, give consideration to both the low-boiling components and the high-boiling components to obtain a suitable peak time distribution.
Detectors
| Detector | Type | Support gases | Selectivity | Detection | Dynamic | |
| limit | Range | |||||
| Flameionization(FID) | Mass flow | Hydrogen and air | Most organic compounds | 100 pg | 107 | |
| Thermal conductivity (TCD) | Concentration | Reference | Universal | 1 ng | 107 | |
| Electron capture(ECD) | Concentration | Make-up | Halides, nitrates, nitriles, peroxides, anhydrides, organometallics | 50 fg | 105 | |
| Nitrogen- phosphorus (NPD) | Mass flow | Hydrogen and air | Nitrogen, phosphorus | 10 pg | 106 | |
| Flame photometric (FPD) |
Mass flow | Hydrogen and air possibly oxygen | Sulphur, phosphorus, tin,arsenic, selenium,boron,germanium,chromium | 100 pg | 103 | |
| Photo-ionization(PID) | Concentration | Make-up | Make-up Aliphatics, aromatics, ketones, esters, aldehydes, amines, heterocyclics, | 2 pg | 107 | |
| organosulphurs,some organometallics | ||||||
| MSD | Mass flow | Universal | 1 pg | 107 | ||
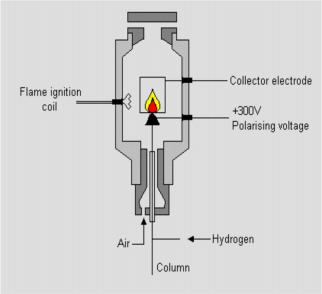
FID Flame Ionized Detector
FID structure
Hydrogen burns in the air to form a hydrogen flame
Organic compounds burn in this flame, generating ions and electrons Attach a strong electric field and a collector above the flame
The ions and electrons will move in the direction of the electric field and form a voltage difference across the collector.
Mass type detector
High sensitivity, low noise, wide linear response range
Simple structure, easy maintenance and good durability
Sample completely burned
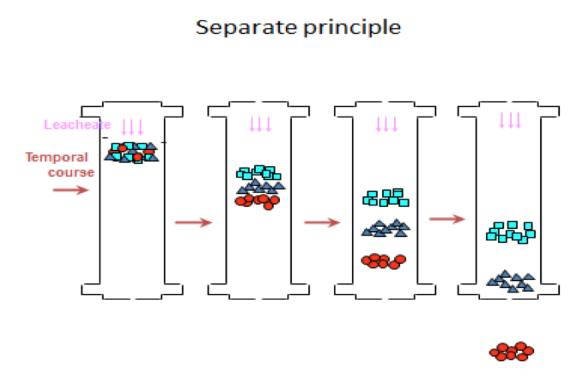
Chromatography Principle
When the sample vaporizes through the injection port and go into the column, the molecules of the compound will dissolve in the stationary phase and then re- evaporate to the mobile phase under the influence of temperature and gas flow. This process is repeated, and the weak property difference is continuously amplified until flow out from the column.
There are two forces that simultaneously affect the separation of components, (1) the vapor pressure balance based on Raoul’s law and (2) the interaction between the component molecules and the stationary phase molecules, the order of the outflow is the result of these two forces competing with each other.
Compounds with strong affinity into the stationary phase are difficult to vaporize to the mobile phase, and the elution time of the column (Retention time, Rt) is longer. Retention time also depends on vapor pressure and enthalpy of vaporization, it is closely related to the boiling point; retention time is also significantly affected by column temperature and mobile phase velocity.
Based on the principle of similar compatibility, columns of different polarity are suitable for the separation of compounds of the corresponding polarity.
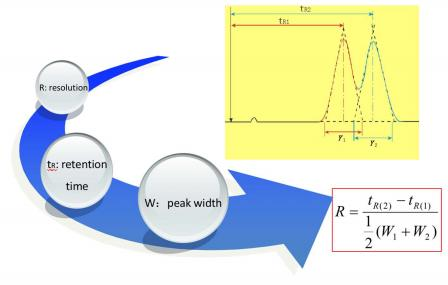
Resolution
times between the two peaks, divided by the combined widths of the elution peaks.
Correction factor
The quantitative analysis of chromatography is based on that the amount of component is proportional to the peak area:
Weight correction factor:
Quantitative method
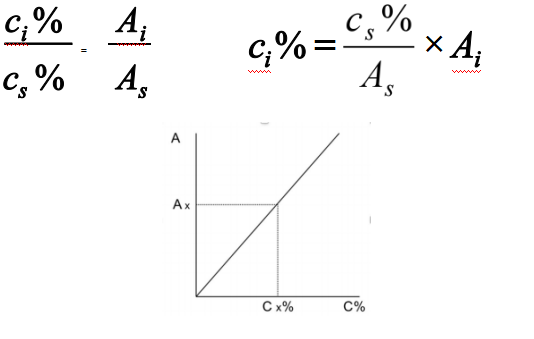
External standard method
Calibration curve can be established according to following equations:
Applied to regular analysis
Advantages: easy operation and calculation
Shortage: the accuracy of results depends on the repeatability of injection and stability of operating conditions. Quantitative injection is required for this method.
Internal standard method
Add certain amount of pure substance into the sample as internal standard.
Application range: only determine several components of the sample, and not all the peaks of components can be flowed
Advantages: less influence of operating conditions, much preciser, and less restrictions than normalization method, fit for trace analysis
Shortage: unfit for rapid analysis
Experiment
Instrument and reagents
G5 Gas chromatograph
Column: DB-1, 30m* 0.25mm* 0.25μm
Carrier Gas: Nitrogen
Auxiliary gas: Air and Hydrogen
Syringe: 5u
Reagents
AR grade absolute ethanol; n-butanol; 2-butanol; 2-methyl-1-propanol; 2-methyl- 2-propanol
Qualitative calibration solution: Take 0.5 mL of four butanol isomer standards in four 10 mL volumetric flasks and dilute with absolute ethanol to the mark (Laboratory provided)
Quantitative standard solution: student preparation Unknown sample solution, n- butanol content is known to be 0.4050g/10mL provided by the laboratory
Experiment procedures
1 According to the operating manual to control the chromatograph normal operation, and set a preliminary analysis conditions.
2 The preparation of quantitative standard solution: take 0.5mL four butanol isomers standard sample in the same 10mL volumetric flask. In the flask, each standard sample must be accurately weighed (accurate to 0.1 mg), and add absolute ethanol to the mark.
3. The optimum chromatographic conditions (column temperature): Inject 1μL mixed standard solution to test the chromatographic conditions, and the peak condition of each column temperature (at least three temperatures) is recorded, and calculate the resolution between the 2nd and 3rd components under different temperature.
4. Relative correction factor: Accurately inject 1 μL of mixed standard solution vy a microsyringe. Repeat the determination three times under the optimal chromatographic conditions. Record the retention time and peak area. If you notice a significant change between the two, repeat more times
5. Unknown sample measurement: Accurately inject 1 μL unknown sample solution by a microsyringe, inject the sample under the above chromatographic conditions, repeat the measurement three times, and record the retention time and peak area. If you notice a significant change between the two, repeat it more times.
6. Qualitative calibration: Inject 1 μL standard mixed solution, and the retention time of each isomer was recorded under the above chromatographic conditions.
7. Turn off the hydrogen and air. when the inlet/detector temperature on the instrument is less than 50°C, shuts off the power to the host, turns off the carrier gas, and shuts down the computer.