1 Scope
This standard specifies the method of testing Chlorpyrifos residue in foods by gas chromatography- flame photometric detector (GC -FPD) and gas chromatography -mass spectrometry (GC -MS).
This standard is applicable to the determination and confirmation of residue content of Chlorpyrifos in corn, brown -rice, scallion, spinach, chili, orange, apple, pine -nut kernel, tea, honey, fish, honey, pork, chicken kidney and chicken liver.
2 Principle
Orange or other vegetal sample is extracted with ethyl acetate. cleaned up by carbon ENVI -Carb column coupled with one florisil solid phase extraction (SPE) column, and determined by GC with external standard method. Pork or other animalized sample is extracted homogenously with water -acetone. The extract is partitioned with dichloromethane is cleaned up by sequentially passing through gel permeation chromatography (GPC), and one active carbon ENVI -Carb column coupled with one florisil solid phase extraction (SPE) column. then the final solution is determined by GC with external standard method. The positive sample should be confirmed by GC -MS.
3 Reagents and materials
All the reagents used should be analytically pure unless otherwise specified. “Water” is double distilled water.
3.1 Acetone: residue grade.
3.2 Dichloromethane: residue grade.
3.3 Cyclohexane: residue grade
3.4 Ethyl acetate: residue grade.
3.5 n-Hexane: residue grade.
3.6 Sodium chloride aqueous solution.
3.7 Anhydrous sodium sulfate: ignited at 650 °C for 4h, and then stored in tightly sealed container.
3.8 Sodium chloride aqueous solution (5%): weigh sodium chloride 5.0g. Dissolve with water and dilute to 100 mL in calibrated flask.
3.9 Ethyl acetate-n-Hexane(1+1V/V) volume 100 mL Ethyl acetate into 250 mL Erlenmeyer flask. then add 100 mL n-Hexane, mix them.
3.10 Ethyl acetate-Cyclohexane (1+1V/V): volume100 mL Ethyl acetate into 250 mL Erlenmeyer flask, then add 100 mL Cyclohexane, mix them.
3.11 Chlorpyrifos standard (C9 H11C13 NO3 PS, CAS No. 2921-88-2) Purity > 98%.
3.12 Standard stock solution: accurately weigh certain amount of Chlorpyrifos standard and dis – solve it with a small volume of ethyl acetate. Dilute with Ethyl acetate to make the standard stock solution of 100 ug/mL. The solution is stored in a refrigerator at 0 C~4 C. Expiry after one year.
3. 13 Standard working solution: dilute the standard stock solution with ethyl acetate to the required concentration to make the standard working solution. The solution is stored in a refrigerator at 0C~4 C. Expiry after six months.
3.14 Florisil SPE tube: Florisil, 500 mg, 6mL, or equivalent.
3. 15 Active carbon SPE tube: ENVI -Carb.250 mg, 6 mL, or equivalent.
3.16 Membrane filter: 0. 45 um.
3. 17 Graphitic carbon: 60 mesh~80 mesh
4 Apparatus and equipment
4. 1 GC: gas chromatography equipped with FPD, 526 nm.
4.2 GC-MS: equipped with electro -Impact source (El).
4. 3 Centrifuge: 4 000 r/min.
4.4 GPC: equipped with isocratic pump and fraction collector.
4. 5 Homogenizer.
4. 6 Rotary vacuum evaporator.
4. 7 Stoppered Erlenmeyer flask: 250 mL
4.8 Separatory funnel: 250 mL
4.9 Concentrate bottle: 50 mL and 250 mL
4. 10 Electronic balance: accurate to 0. 000 1 g.
4. 11 Plastic centrifuge tube: 50 mL
5 Preparation and storage of test sample
5.1 Preparation of test sample
5.1.1 Corn, brown-rice, tea, pine nut kernel, peanut and Honey
Take approximately 500 g of representative sample. Smash thoroughly by a breaker. Mix thoroughly. Put in clean containers. Seal and label them.
5.1.2 Orange, apple, spinach, and scallion
Take approximately 500 g of representative sample. Collect the edible parts (do not wash with water) and cut into minces. Crush with a crusher into pulp. Mix thoroughly. Put in clean containers. Seal and label them.
5.1.3 Pork. chicken liver, chicken kidney. and fish
Take approximately 1 kg of representative sample. Collect the edible pieces. Crush with a crusher. Mix thoroughly. Put in clean containers Seal and label them
5.1.4 Chili
Take approximately 500 g of representative sample. Mix thoroughly. Put in clean containers. Seal and label them.
5.2 Storage of test sample
The test samples of cereals, nuts, tea. honey and chili should be stored between 0°C~ 4° C. Other samples should be frozen and stored below -18 ° C. While sampling and preparing sample, please avoid contamination or any factors that may change residue content.
6 Procedure
6.1 Extraction
6.1.1 Orange, apple, spinach, scallion, corn, brown -rice, and chili
Weigh 10g (accurate to 0.01g) of the test sample into a 50 mL centrifuge tube (4. 11). Add 6 g of anhydrous sodium sulfate and 30 mL ethyl acetate. Homogenize for 2 min. Centrifuge for 5 min at 5000 r/min. Filter the supernatant into a 250 mL concentrate bottle. Extract the residue with 30 mL of Ethyl acetate once more and filter the second supernatant. Combine the filtrates. Condensed to nearly dry by rotary evaporator in 40 °C water bath. Add 2 mL of ethyl acetate-n-Hexane (1+1) to dissolve the residue and then wait for cleaning -up.
6.1.2 Tea
Weigh 5g (accurate to 0.01 g) of the test sample into a 50 mL plastic centrifuge tube. Add 10 mL water and 30 mL ethyl acetate. Homogenize for 2 min. Centrifuge for 5 min at 5,000 r/min. Filter the supernatant into a 250 mL concentrate bottle. Extract the residue with 30 mL of Ethyl acetate once more and filter the second supernatant Combine the filtrates. Condensed to nearly dry by a rotary evaporator in 40 °C water bath. Add 2 mL of ethyl acetate-n-Hexane (1+1) to dissolve the residue and then wait for cleaning -up.
6.1.3 Pork, chicken liver, chicken kidney, fish, pine -net kernel and peanut
Weigh 20 g (accurate to 0.01g) (5g for pine -net kernel and peanut) of test sample into a 250 mL stoppered Erlenmeyer flask. Add 20 mL water and 100 mL acetone. and homogenize for 3 min. Filter the supernatant into a 250 mL concentrate bottle. Extract the residue with 50 mL of acetone once more and filter the second supernatant. Combine the filtrates. Condensed to about 20 mL by a rotary evaporator in 40 °C water bath.
Transfer the extract into one 250 mL separatory funnel. Add 100 mL sodium chloride aqueous solution and 100 mL dichloromethane. Shake 3 min. Stand still waiting for layering. Collect dichloromethane phase. Extract aqueous phase twice with 2 x 50 mL dichloromethane. Combine dichloromethane phases. Dehydrate with an anhydrous sodium sulfate tube, Collect eluates into one 250 mL concentrate bottle. Condensed to nearly dry by a rotary evaporator in 40 °C water bath. Add 10 mL of ethyl acetate -n-Hexane (1+1) to dissolve the residue, filter the solution with 0. 45 μ m membrane and then wait for cleaning -up.
6.2 Cleaning -up
6. 2. 1 GPC Cleaning -up
6.2. 1. 1 GPC operating condition
a) GPC column: 700 mm x 25 mm (i. d.) Bio Beads S-X3 or equivalent;
b) Mobile phase: cyclohexane -ethyl acetate (1+1)
c) Flow rate: 4. 7 mL/min:
d) Injection volume at sample loop: 10mL:
e) Time of pre-rinsing: 10 min:
f) GPC balance time: 5 min:
g) Time of collecting the eluates: 22 min~31 min. 6.2.1.2 GPC cleaning-up procedure Purify 10 mL of the solution which is waiting for cleaning -up (6. 1. 3) according to the condition described in the section 6. 2. 1. 1. Condense the collected constituents to nearly dry at 40 °C. Dissolve the residue with 3 mL of ethyl acetate -n-hexane (1+1) and wait for next SPE cleaning -up(pine-nut kernel and walnut kernel should repeat GPC cleaning -up step described in the section 6.2.1.1) 6.2.2 SPE Cleaning -up Couple the active carbon SPE tube and florisil SPE tube up to down (for samples containing much colorants, such as spinach, tea etc, add 1.5 cm high graphic carbon SPE tube (3. 16) in the active carbon tube), Rinse the coupling columns with 6 mL of n -hexane-ethyl acetate (1+1) in advance. Discard the washings. Transfer 2 mL of the sample solution from 6. 1. 1, 6. 1. 2 and 6. 1. 3 into the upper column, Elute with 3 mL of n-hexane-ethyl acetate (1+1). Collect eluates into a concentrate bottle. Evaporate to nearly dry in water bath. Dissolve the residue and dilute exactly to 1.0 mL with ethyl acetate for determination by GC or confirmation by the GC-MS. 6.3 Determination 6.3.1 GC conditions
a) Chromatographic column: HP -5 silica capillary column, 30 m x 0. 25 mm (i. d. ) film thickness 0. 25 u m, or equivalent:
b) Column temperature: 50 °C (hold 1 min) (30°C/min) – 180 °C (hold 1 min) (10° C/min) – 250°C (hold 10min).
c) Injection port temperature: 250 C:
d) Interface temperature: 250 C:
e) Carrier gas: Nitrogen, purity > 99.999%, flow rate 1.0 mL/min:
f) Injection volume: 1 μL;
g) Injection mode: splitless.
6.3.2 GC -MS operating conditions
a) Chromatographic column: HP -5 silica capillary column. 30 m x 0. 25 mm (i. d.) x 0. 25 um, or equivalent
b) Column temperature: 50 °C (hold 2 min) (20°C/min) – 200 °C (hold 1 min) (5° C/min) – 270°C (hold 18min).
c) Injection port temperature: 280°C
d) Interface temperature: 280 °C:
e) Carrier gas: Helium. Purity > 99. 999%. flow rate 1.0 mL/min;
f) Injection volume: 1 uL
g) Injection mode: splitless. Open the valve after 1. 2 min:
h) Electron ionization mode: El:
i) Ionization energy: 70 eV:
j) Determination mode: SIM;
k) Selected monitoring ions (m/z); quantiation ion is 197 and confirmation ions are 258, 286, 314:
l) Solvent delay 9. 0 min.
6.3.3 GC determination
According to the approximate content of Chlorpyrifos, select one standard working solution which has similar concentration of the sample solution. The responses of Chlorpyrifos in the standard working solution and in the sample solution should be within the linear range of the instrumental detection. Qualification is undergone by retention time; quantitation is undergone by the comparison peak areas between sample and standard work solution.
7 Detection Limit and Recovery
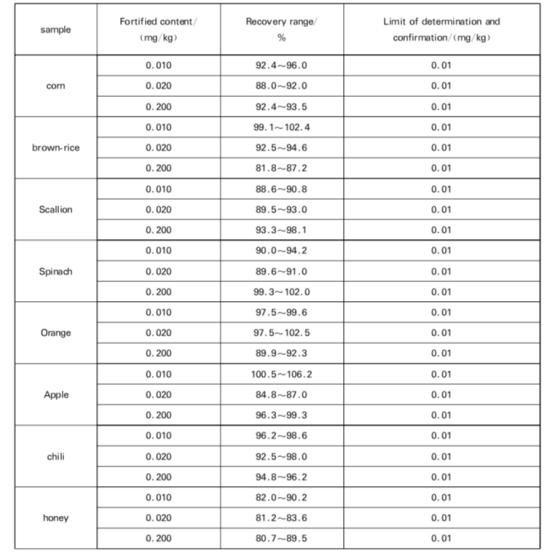
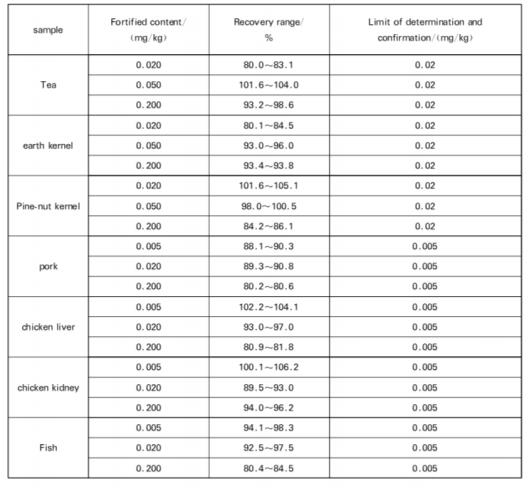
7.1 Limit of Determination and Confirmation
The method detection and confirmation limit are shown in the Table.
7.2 Range of fortification and recovery
The range of fortification and recovery of this method is shown in the Table.
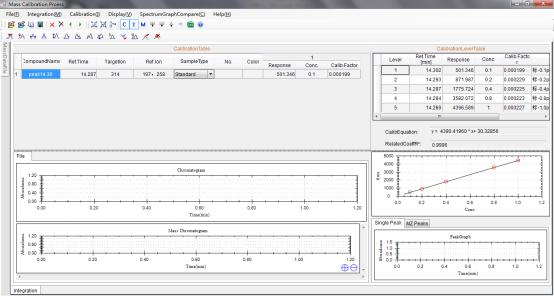
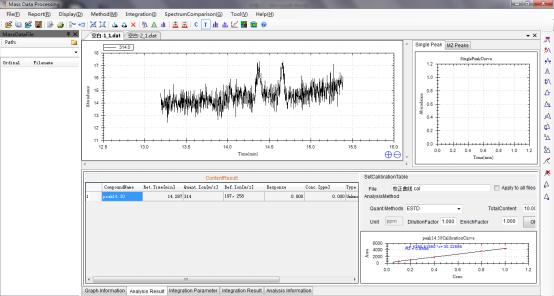
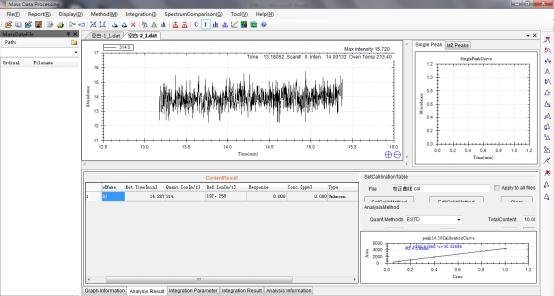
8 Example Data of Testing Chlorpyrifos in Corn by GCMS.
8.1 Calibration curve of Chlorpyrifos generated by Standards.
8.2 Corn sample concentration measured through the calibration curve.
8.2.1 Corn Sample 1
8.2.2 Corn Sample 2