Introduction
This application note describes the method of testing the concentration of Arsenic and Selenium simultaneously in Wastewater by use of Persee’s Heavy Metal Analyzer (PF7).
Equipment and Reagent
Equipment:
1. Heavy Metal Analyzer with 2 or more lamp slots.
2. As and Se Hollow Cathode Lamp.
3. Hot Plate.
Reagent:
4. HNO3 – HClO4 digestions liquid (50mL HNO3 and 50mL HClO4 mixed together).
5. KBH4 Solution (15g/L): 1.25g KOH dissolved in water, then add 3.75g of KBH4 and dilute to 250mL. This solution must be prepared fresh daily.
6. Thiourea-Ascorbic acid solution: dissolve 1g thiourea in water; add 1g ascorbic acid into the solution. Dilute to 100mL and mix well.
7. HCl solution (5%): 5% HCl dissolved in water.
8. As Standard Solution (20mg/L): transfer 10mL of 100mg/L As solution into 50mL volumetric flask, dilute to 50mL and mix well.
9. Se Standard Solution (5mg/L): transfer 1.00mL of 500mg/L As solution into 100mL volumetric flask, dilute to 100mL and mix well.
10. As and Se Standard Solution (200 g/L As and 50 g/L Se): transfer 1.00mL of As Standard Solution (20mg/L) and 1mL of Se Standard Solution (5mg/L) together into a 100mL volumetric flask, dilute to 100mL and mix well.
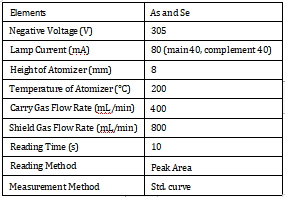
Equipment Condition
Instrument Working Parameters:
Table 1. Instrument Working Parameters.
Experiment Procedure
Standard Curve:
Transfer 0, 0.5, 1.0, 2.0, 3.0, 4.0, 5.0mL prepared As and Se standard solution (200 g/L As, 50 g/L Se) into 7 different 25mL volumetric flask. Add 2mL HCl (guarantee reagent) solution and mix well. Add 5mL thiourea-ascorbic acid solution. Dilute to the 25mL marker, mix well again. Let solution sit for 30min. Use the prepared KBH4 Solution (15g/L) as Reducer; and HCl solution (5%) as Carrier Liquid. Run the experiment by PF7.
Wastewater Sample Preparation:
Transfer 50mL wastewater sample into a 150mL tall beaker, then add 5mL of HNO3 – HClO4 digestions liquid. Put the tall beaker on a hot plate until white smoke appears around the bottleneck. Remove the beaker from the hotplate and wait for it to cooldown. Add 5mL of HCl (1+1), then continue heating on the hot plate until the yellowish-brown smoke disappears. Remove the beaker from the hotplate and wait for it to cooldown. Transfer the sample into a 25mL volumetric tube with small amount of pure water. Then, add 2mL HCl (guarantee reagent) mix well. Add 5mL thiourea- ascorbic acid solution. Dilute to the 25mL marker, mix well again. Let the solution sit for 30min.
Instrument Procedure:
Use the prepared KBH4 Solution (15g/L) as Reducer; and HCl solution (5%) as Carrier Liquid.
Results
By using the method of HNO3 – HClO4 digestion, interference is limited, thus the result is accurate and reliable. The sensitivity of the experiment is high. The detection limits ofAs and Se are 0.89g/L and 0.5 g/L. The RSD value of the mixed standard solution (24 g/L As and 6 g/L Se) are 3.5% and 1. 1% and the Recovery rates are 91.5%~101.2% and 94%~100.5% for As and Se respectively.
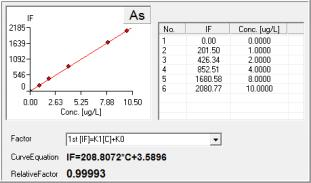
As Results by PF7:
Standard Curve:
As Standard Graph at 10 g/L:
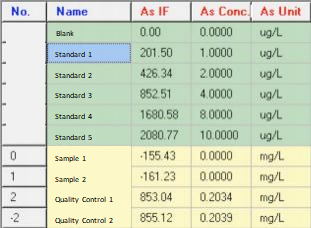
As Quality Control 1:
As Quality Control 2:
As Sample 1:
As Sample 2:
As Final Results:
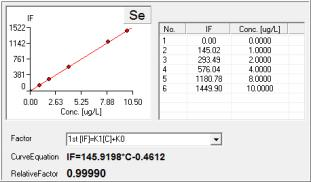
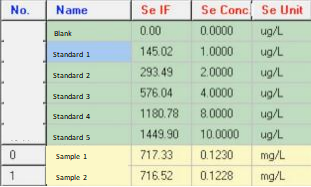
Se Results by PF7:
Standard Curve:
Se Standard Graph at 10 g/L:
Se Sample 1:
Se Sample 2:
Se Final Results: